Quantum Mechanics
The fundamental rules of Quantum Mechanics are very deep. For our understanding on Atoms and electrons, it can be described like this - Quantum Mechanics speaks on the arrangement of electrons in an atom in terms of its energy levels, energy subshells and its orbitals (space occupied by an electron within an atom). Let us understand what this means in detail.
Louis de Broglie, a French physicist came up withan altogether new and fascinating idea of electron. He said that every particle, be it an electron, atom or a tennis ball has a wave like property. Consequently, electrons sometimes behave like waves and other times like particles. This nature of is known as wave-particle duality.
This wave like behavior changes the whole concept of electron orbits. Unlike previous models where electrons keep orbiting the nucleus, here electrons are present in a wave formed around the nucleus. These were standing or stationary waves. You can visualize this as a guitar string struck, creates a stationary wave. Here no particles are moving but the guitar string resembles a wave. Now imagine this orbit like waves formed around the nucleus.
Warner Heisenberg, another German Physicist proposed the famous uncertainty principle which states “It is impossible to define what time and where an electron is and where is it going next. This makes it impossible to know exactly where an electron is traveling in an atom.
With two observations in place, Erwin Schrodinger, an Austrian Physicist derived a set of mathematical equations to predict the probable location of electrons within an atom. These probable locations around the nucleus were known as Orbital. These orbitals are the most probable locations where an electron can be found. Imagine you take the photograph of an electron at a precise location, based on Schrodinger equation. Now you have a location pictured. Suppose you continue taking a series of pictures of electron location and then superimpose each of those pictures, you end up with an image which resembles a cloud of electrons in a certain orbital, as shown in the figure. The area with the densest cloud means the greatest probability of finding an electron (also known as electron density cloud).
These observations also followed laws such as - only two electrons can share an orbital, one spinning clockwise and the other spinning anticlockwise. Each of these orbital have different shape and is termed as s-orbital, p-orbital, d-orbital and f-orbital based on their shape. Each of these orbitals can have different levels. s-orbital has a single level, p-orbital = 3 levels, d orbital = 5 and f-orbital = 7 levels. Based on those levels, orbitals at any particular level are distinguished by their names like 1s, 2s, 3s…, 2px, 2py, 2pz… (x, y, z to distinguish between each level).
s-orbital
Suppose you had a hydrogen atom with 1 Electron and 1 Proton and you plot the position of an elec tron at that instant. Again you repeat this task and find that the electron is in another position. You plot it again. If you keep repeating this task, you get a map of all the locations the electron is likely to be found. Now you get to know that electron can be found anywhere around the nucleus in a spherical shape. This region of space was known as 1s, where “s” signifies the shape and 1 denotes the energy level of the orbital. Similarly 2s (3s, 4s) is the next energy level in s-orbital where there is a possibility of finding an electron.
tron at that instant. Again you repeat this task and find that the electron is in another position. You plot it again. If you keep repeating this task, you get a map of all the locations the electron is likely to be found. Now you get to know that electron can be found anywhere around the nucleus in a spherical shape. This region of space was known as 1s, where “s” signifies the shape and 1 denotes the energy level of the orbital. Similarly 2s (3s, 4s) is the next energy level in s-orbital where there is a possibility of finding an electron.

p-orbital
After the s-orbital, there is another orbital called p-orbital. This resembles number “8” (imagine a three dimensional 8), where there is a central nucleus and two locations above and below it where the electron can reside (number 8 for visualization purpose, they can even look like “8”). At any one energy level, there can be three different p-orbital at right angles to each other and are commonly known as 1px, 1py and 1pz. In the next energy level of p-orbital, there can be subsequent levels like 2px, 2py, 2pz (3px, 3py, 4px, 4py, 4pz…)

d-orbital and f-orbital
There are two other orbitals where electrons can occupy apart from s and p. They are the d and f orbitals. d-orbitals can have 5 orbitals and f-orbitals can have seven in each of them.
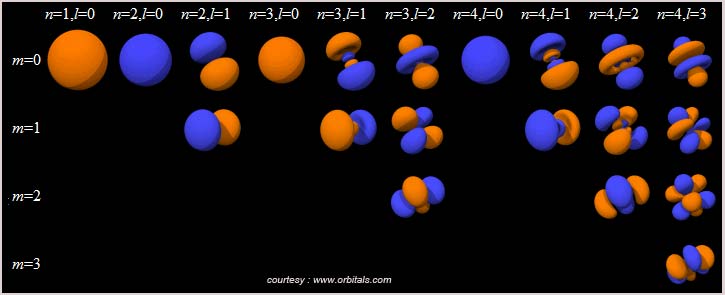
www.orbitals.com has a nice orbital viewer which is been provided for free. You need to download and install this.
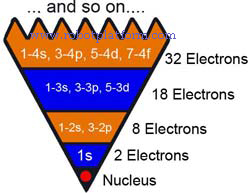
If the above concept is confusing, consider a huge pyramid built upside down, as given in the figure. Imagine each division has certain number of rooms. The ground floor is occupied by the nucleus. First floor occupied by two 1s electron. Second floor can occupy 8 electrons (2s, 2px, 2py, 2pz). Third floor is built to contain 18 electrons (One 3s orbital with 2 electrons, three 3p-orbital [with 6 electrons], and five 3d-orbital [with 10 electrons]) and the forth floor accommodating 32 electrons [one 4s, three 4p, five 4d and seven 4f].
Atomic number of an atom defines the number of Protons within an atom (also called Proton Number).
Conclusion:
If you have skipped reading the entire description, then this will give you a brief picture on Atomic theory. However, I suggest that you read the complete tutorial whenever possible.
An Atom contains three particles. The central nucleus contains protons with positive energy, and neutrons with no charge. Electrons with a negative charge stay around the nucleus (call it orbit, electron cloud… whatsoever; just visualize it). If an atom loses an electron then it is positively charged, as it loses a negatively charged electron. Similarly if it gains an electron, then an atom becomes positively charged. If it gains or loses an electron, then the atom is called a positive or negative ion respectively.
Electrons occupy discrete shells with fixed maximum number of electrons per shell, each with different energy levels. The electrons in the outer most shell of an atom determine the electrical and chemical characteristics of an atom and this is known as valence shell with least energy level.
We know that each shell can contain a maximum number of electrons. Suppose the third orbital requires 18 electrons and only 4 electrons occupy, then the empty space which do not have an electron is know as an electron hole, or simply a hole. (Hole is absence of an electron)
If everything is made of protons, electrons, neutrons, then why are all objects or elements not similar?
A dissimilar number and arrangement of these particles within the atom and the atom structure gives different characters and shapes to an object.
This ends a basic understanding of Atomic theory. As usual, comments are always welcome.
Atom and its relation with electricity, Volt, Power are are further explained in "Electricity Tutorial"
Do you have anything to say?
Visit the Forum to discuss, learn and share anything related to robotics and electronics !!








