Batteries for Robots
What are batteries?
Who doesn’t know what a battery is? They are everywhere; wall clocks, wristwatches; calculators, mobile phones, flashlights, cars, motorbikes and most other electrical gadget you can imagine.
To answer the above question, a battery is a device which converts chemical energy into electrical energy. As most of our robots use batteries, we will spend some time understanding what a battery is, and the different types of batteries available. Later we will understand which battery best suits our robots.
How batteries work
Simply speaking, batteries are chemical containers. Electrochemical reaction in these chemicals produces electrons and the flow of these electrons from one terminal to another leads to flow of electricity. The reaction of losing electrons is known as Oxidation and absorbing electrons is known as reduction.
Now let us go in detail and realize the fundamentals of batteries.
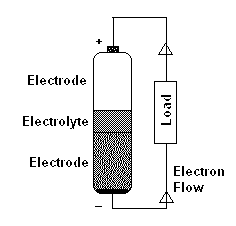 Typical batteries have two terminals. The terminal which is marked (+) is generally known as Positive or Cathode and the terminal marked (-) is known as Negative or anode (However, I suggest you to avoid using cathode and anode and simply refer it as Positive terminal or negative terminal). These two parts of a battery make up the electrodes and are physically divided by a separator called the electrolyte.
Typical batteries have two terminals. The terminal which is marked (+) is generally known as Positive or Cathode and the terminal marked (-) is known as Negative or anode (However, I suggest you to avoid using cathode and anode and simply refer it as Positive terminal or negative terminal). These two parts of a battery make up the electrodes and are physically divided by a separator called the electrolyte.
When a circuit connects these two terminals, Oxidation reaction happens at the negative terminal releasing electrons. On the other terminal reduction reaction happens which absorbs electrons that are released from the negative terminal. This flow of electrons from negative terminal to the positive terminal results in electricity (DC).
The entire process is setup in a case and is known as a cell. If two or more cells are electrically connected, only then they are known as a battery. But it is universal to call a “Cell” as a battery and we will continue using the term “Battery” to avoid further confusion. For example a 1.5V AAA cell is single cell, but it is generally referred to as a battery.
Battery performance parameters
Each battery is designed to fulfill a specific task and no battery can meet all of your robot requirements. Batteries are designed to be either high power, or high energy and rarely some batteries for high durability. I have listed few terminologies which can help you further in deciding the right battery for your robot requirement.
Terminal Voltage: The voltage between battery terminals is called the terminal voltage, measured in Volts. Generally a cell has a voltage ranging from 1 to 2 volts (what if researchers use Fluorine gas with reduction potential of 2.87 and lithium metal with -3.05 to get 5.92 volts? just a wild guess…). Higher voltages can be obtained by connecting several batteries (actually cells) in series.
Open circuit voltage: When the battery is neither charging nor discharging, then the terminal voltage is referred as Open circuit voltage.
Voltage curve: Voltage curve of a battery provides a graphical representation of progressive voltage drop as it discharges.
Discharge Curve: Most batteries have a tendency to droop in voltage with usage. Few batteries maintain their initial voltage until they die. This discharge in voltage is graphically represented against time. Flatter the curve, better the battery; most primary batteries have a sloping discharge and secondary batteries tend to have a flat discharge curve.
Storage capacity: This is the amount of current a battery can supply for a unit of time measured in Ampere-hour (for batteries it is generally milliamp-hour). For example if a battery is rated 2000mAh, then the battery can supply 2 ampere or 2000 milliamps of current for one hour. If the robot is consuming only 1000mA of current, then your battery runs for 2 hours. Now you should know the difference between mA and mAh. Generally all batteries have mAh printed on them; higher current output can be obtained by connecting batteries (actually cells) in parallel.
C-rate: This is not very important for a robot designer, but still… C-rate is the charging and discharging rate of a battery, expressed in terms of its storage capacity which is generally mAh or Ah. 1C would mean discharging all the stored energy in 1 hour, and 0.5C would mean discharging all the energy in 2 hours. For example, a battery rated 1.5mAh provides 1500 milliamps of current for one hour if it is discharged at 1C rate. If the same battery is rated as 0.5C, then it discharges at 750mA for 2 hours. Generally most batteries are rated at 1C.
Energy density: Energy density is the amount of energy stored in the battery per unit volume.
Power: This is the amount of power in a battery per unit volume measured in Watts/m^3.
Number of cycles: Number of times a battery can be recharged and discharged (applicable for reachable batteries) before the performance drops below expected level.
Shelf life: The length of time a battery can stay fit on the shelf/store without being used, i.e. unused.
Lifespan: The length of time before the battery performance degrades; either used or unused.
Temperature: Performance of most batteries degrades with change in temperature. It is always good to select a battery which does not degrade in performance with a little change in temperature.
Battery chemistry: Different manufacturers use different chemistry to make batteries. Some of them might use toxic components and some might emit dangerous gases while charging or discharging. The best of all would be the one which is environmentally friendly.
Cost & Size: Cost of a battery is one of the most important parameter when considering a battery. We do not want to invest 100$ when we plan to build a 50$ robot. On the other hand, size and weight of a battery does matter; we cannot add a battery weighing 10 pounds into a tiny quad rotor, or a 2 feet long battery into a small mobile robot.
Depth of Discharge (DOD): This is the measure of how deeply a battery is discharged. For example, 40% DOD means that we have utilized 40% of the battery and 60% is remaining if the capacity of the battery is 100%. Be informed that most batteries are not designed for 100% discharge.
Internal resistance: All batteries exhibit an internal resistance which affects its state of charge. As internal resistance increases, battery generates more heat decreasing thermal stability and affecting battery efficiency.
Battery Memory effect: Also known as lazy battery effect is generally found in few types of rechargeable batteries (especially nickel cadmium batteries). If batteries are charged when they are not fully discharged, they somehow remember the previous discharge point and needs a charge whenever it hits that particular point. For example, if a battery discharges to 50% and you recharge it, next time it wouldn’t run below the 50% mark even if you wanted it to discharge lower.
There is no ideal battery which acts at all conditions. Select a battery that best suits your robot based on above parameters.
In the next section we will discuss about the different types of batteries available, their advantages, disadvantages and usage.
Do you have anything to say?
Visit the Forum to discuss, learn and share anything related to robotics and electronics !!








